

For these reasons research on novel methods of laboratory diagnosis is underway, and research has also aimed to see if the somewhat complicated two-tier test algorithm can be simplified without significantly impacting on test performance.
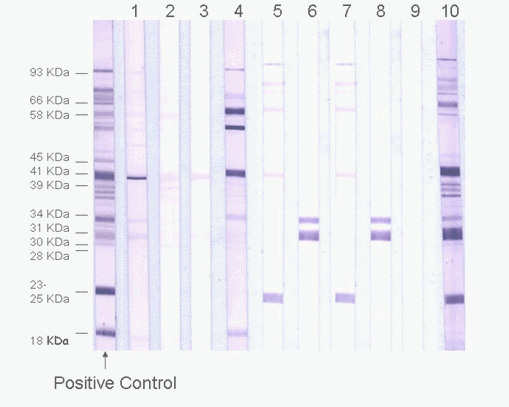
In early disseminated Lyme disease some patients may also test negative, although with progression of infection to late disseminated Lyme disease, test sensitivity increases. However for early Lyme disease cases with typical cutaneous Erythema migrans (EM) lesions, sero-diagnosis is not needed. Using CDC Western blot interpretation criteria, the two-tier test has some limitations to specificity as false-positivity has been detected associated with cross-reactivity to other infections, and in early Lyme disease specific antibody levels are low so test sensitivity is low (ca 30%). Sensitivity of this two-tier algorithm has been questioned by some patient groups and medical practitioners, but alternative interpretation criteria used by some private laboratories in the US have high (>50%) false positive rates and patients may be being falsely diagnosed as having “chronic Lyme disease”. This algorithm, when using specific interpretation criteria, is the method recommended by infectious disease experts and public health organisations such as US Centres for Disease Control & Prevention (CDC) and the Public Health Agency of Canada. The most frequently used laboratory method for Lyme disease diagnosis is serology using the two-tier (Enzyme Immuno Assay followed by Western blot ) algorithm. In North America Lyme disease is caused by the tick-borne bacterium Borrelia burgdorferi sensu stricto, (hereafter shortened to B.
burgdorferi strain variation in Canada, and further studies are needed to explore this. Geographic and interannual variations in the prevalence of samples testing positive may be consistent with B.

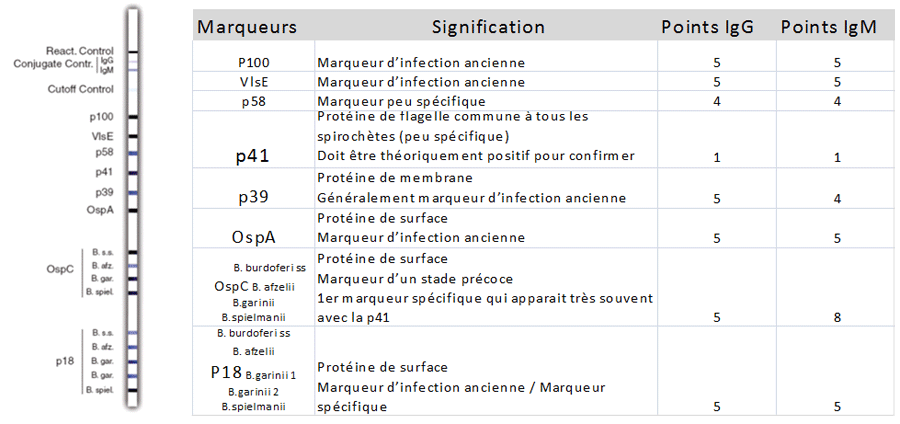
These findings suggest that the current two tier test may not be simplified and continued use of the current two-tier test method and interpretation is recommended. In most cases the prevalence of samples testing positive were highest in Nova Scotia, and lower in samples from Manitoba westwards. Significant variations were found amongst years and geographic regions in the prevalence of samples testing positive using the overall IgG WB algorithm, and for individual proteins of the algorithm. No one protein was highly concordant with the IgG WB result. Geographic and interannual variations in proportions of samples testing positive were explored by logistic regression. Metrics of relative sensitivity, specificity and the kappa statistic measure of concordance were used to assess the capacity of responses to individual proteins to predict the overall IgG WB result of 2524 EIA (C6)-positive samples from across Canada. Because Borrelia burgdorferi strains vary geographically in Canada, geographic variations in serological responses were also explored. In this study, accuracy of individual proteins of the IgG WB algorithm in predicting the overall test result in samples from Canadians was assessed. Simplification of this algorithm would be advantageous unless it impacts test performance. Lyme disease is emerging in eastern and central Canada, and most cases are diagnosed using the two-tier serological test (Enzyme Immuno Assay followed by Western blot ).


 0 kommentar(er)
0 kommentar(er)
